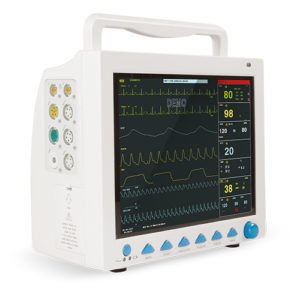
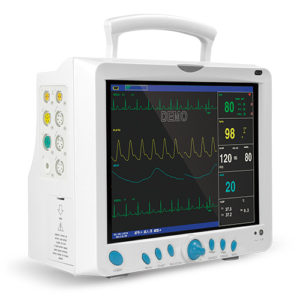
MS200 NIBP Simulator
$1,490.00
– Multi-purpose test instrument for oscillometric Non-Invasive Blood Pressure (NIBP) Monitors
– Simple and convenient operation with a built-in pump
– Generates pressures up to 400 mmHg (53.3 kPa)
– Offers dynamic blood pressure simulations and static calibration
– Functions for pressure gauge testing, leak testing, and relief valve testing
– Simulates conditions for adult, neonate, and user-defined patients
– Includes arrhythmias and respiratory artifact simulations
– Features a built-in air chamber for both adult and neonate cuffs
– Equipped with a vibrant 4.3″ LCD display with 65k colors
– Ideal for accurate analysis of various NIBP Monitors
MS200 NIBP Simulator
Professional Multi-Purpose Blood Pressure Monitor Testing Instrument – Advanced Oscillometric NIBP Calibration and Quality Assurance System
Fast Worldwide Shipping
ISO Calibrated
24/7 Expert Support
Professional Grade





Clinical Applications
Technical Specifications
| Product Model | MS200 NIBP Simulator |
| Manufacturer | Contec Medical Systems |
| Display | 4.3″ High-Resolution LCD (65,000 Colors) |
| Maximum Pressure | 400 mmHg (53.3 kPa) |
| Pressure Accuracy | ±1 mmHg (Calibrated to NIST Standards) |
| Pump System | Built-in Internal Pump |
| Air Chamber | Internal Chamber (Adult and Neonate Simulation) |
| Patient Types | Adult and Neonate |
| Cuff Compatibility | Arm and Wrist Cuffs |
| Predefined Scenarios | 8 Standard Variation Patterns |
| Custom Simulations | User-Defined Blood Pressure Values |
| Arrhythmia Simulation | Multiple Cardiac Rhythm Patterns |
| Artifact Simulation | Respiratory Motion Artifacts |
| Test Functions | Dynamic BP, Static Pressure, Leak Test, Relief Valve, Pressure Gauge |
| Leak Detection Range | 0-10 mmHg/minute |
| Data Storage | Test Results Memory |
| Power Supply | AC Adapter (100-240V) + Rechargeable Battery |
| Battery Life | 8+ Hours Continuous Operation |
| Connectivity | USB Port for Data Export |
| Operating Temperature | 5°C to 40°C (41°F to 104°F) |
| Storage Temperature | -20°C to 55°C (-4°F to 131°F) |
Comprehensive Testing Functions
| Dynamic Blood Pressure Simulation | Generates realistic oscillometric waveforms simulating systolic, diastolic, and mean arterial pressures with physiologically accurate pulsatile variations |
| Static Pressure Calibration | Precision static pressure output for verifying NIBP monitor pressure transducer accuracy and calibration at multiple pressure points |
| Automated Leak Testing | Quantifies pneumatic system leak rate in mmHg/minute to identify deteriorated tubing, valve defects, or connection failures |
| Pressure Relief Valve Testing | Verifies safety overpressure valve activation threshold ensuring patient protection from excessive cuff inflation |
| Pressure Gauge Verification | Tests accuracy of analog pressure gauges used in manual blood pressure monitors and reference standards |
| Adult Simulation Mode | Simulates adult arm compliance and oscillometric pulse amplitude characteristics for standard blood pressure monitor testing |
| Neonate Simulation Mode | Replicates neonatal limb compliance and smaller pulse amplitudes for pediatric and NICU blood pressure monitor verification |
| Arrhythmia Patterns | Simulates irregular cardiac rhythms (atrial fibrillation, PVCs, bigeminy) to test NIBP monitor artifact rejection algorithms |
Key Features & Benefits
High-Accuracy Pressure Generation
The MS200 delivers ±1 mmHg pressure accuracy traceable to NIST standards, providing reference-quality calibration for critical blood pressure monitoring equipment. This exceptional accuracy exceeds most regulatory requirements and manufacturer specifications, ensuring absolute confidence in NIBP monitor verification results. The precision pressure generation system maintains stability across the entire 0-400 mmHg range, from low neonatal pressures to high adult pressures and safety valve testing thresholds.
Realistic Oscillometric Waveform Simulation
Advanced waveform generation produces physiologically accurate oscillometric pulse envelopes that replicate true arterial pulsations. Unlike simple pressure simulators that only provide static values, the MS200 generates dynamic pressure oscillations with correct amplitude, frequency, and morphology. This realistic simulation enables comprehensive testing of NIBP monitors’ oscillometric analysis algorithms, ensuring devices accurately extract systolic, diastolic, and mean pressures from pulse waveforms under various conditions.
Adult and Neonate Patient Modes
Separate adult and neonate simulation modes replicate the distinct biomechanical characteristics of different patient populations. Adult mode simulates the compliance and pulse amplitude of a standard arm, while neonate mode replicates the higher compliance and smaller pulse amplitudes characteristic of infant limbs. This dual capability is essential for hospitals with both adult and pediatric services, allowing verification of NIBP monitors across all patient care areas with a single instrument.
Arrhythmia and Artifact Simulation
Comprehensive simulation of cardiac arrhythmias and respiratory artifacts tests NIBP monitors under challenging conditions that cause measurement errors in clinical use. Arrhythmia modes include atrial fibrillation (irregular rhythm), premature ventricular contractions (PVCs), and bigeminy patterns. Respiratory artifact simulation replicates pressure variations caused by patient breathing during measurement. These challenging test conditions verify that NIBP monitors correctly reject artifacts rather than reporting erroneous blood pressure values.
Eight Predefined Scenarios
Preprogrammed test scenarios cover common clinical blood pressure patterns from hypotension to hypertensive crisis. Predefined scenarios include: normal adult (120/80), hypertension stage 1 (140/90), hypertension stage 2 (160/100), severe hypertension (180/110), hypotension (90/60), normotensive neonate (70/50), and others. These standardized scenarios enable consistent testing across multiple NIBP monitors and facilitate comparison of device performance. Quick access to predefined scenarios accelerates routine preventive maintenance testing.
User-Defined Custom Simulations
Flexible custom simulation mode allows technicians to program any desired systolic, diastolic, and heart rate combination for specialized testing. This capability is invaluable for troubleshooting specific device failures, verifying accuracy at pressures relevant to particular patient populations, or testing manufacturer specifications requiring specific pressure values. Custom simulation accommodates research applications, development testing, and forensic investigation of clinical incidents involving NIBP monitor readings.
Automated Leak Testing
Quantitative leak detection identifies pneumatic system deterioration before it causes clinical measurement failures. The MS200 pressurizes the NIBP monitor’s pneumatic system, seals it, and precisely measures pressure decay over time. Leak rates are reported in mmHg/minute with results compared against manufacturer specifications. This objective leak quantification replaces subjective “inflate and listen” methods, providing documentation of pneumatic integrity for quality assurance records and helping predict when tubing or valve replacement will be needed.
Safety Valve Verification
Critical safety testing verifies that overpressure relief valves activate at correct thresholds, protecting patients from excessive cuff inflation that could cause limb ischemia or nerve damage. The MS200 gradually increases cuff pressure while monitoring for valve activation, documenting the exact pressure at which relief occurs. This safety verification is mandated by many regulatory standards and is particularly important for pediatric NIBP monitors where smaller patients are more vulnerable to overpressure injury.
High-Resolution Color Display
The vibrant 4.3-inch LCD screen with 65,000 colors provides exceptional visibility of test parameters, waveforms, and results. Large fonts and high-contrast color schemes remain legible under challenging lighting conditions from bright operating rooms to dimly lit equipment storage areas. Real-time waveform display shows the oscillometric pulse envelope during simulation, allowing technicians to visually verify waveform quality. The intuitive graphical interface reduces training requirements and minimizes user errors during complex testing procedures.
Integrated Internal Pump
Built-in pump eliminates dependence on external compressed air sources or manual inflation, enabling testing anywhere without support equipment. The internal pump automatically inflates the simulated cuff to test pressure, maintains pressure during measurement, and controls deflation rate—replicating the complete NIBP measurement cycle. This self-contained design makes the MS200 truly portable for field service work, bedside testing, or mobile biomedical engineering services. Pump performance is optimized for quiet operation, minimizing disruption in clinical areas.
Rechargeable Battery Operation
Eight-hour battery life supports a full workday of field testing without requiring AC power access. This cordless capability is essential for servicing NIBP monitors in patient rooms, operating theaters, or remote clinics where finding available power outlets is challenging. The rechargeable battery eliminates ongoing battery replacement costs while providing environmental benefits. Battery status indicator warns when charging is needed, preventing mid-test power loss. Dual power capability (battery or AC adapter) accommodates both mobile field work and stationary bench testing.
Test Results Memory and Data Export
Internal memory stores test results for documentation and trend analysis of NIBP monitor performance over time. USB connectivity enables export of test data to computers for integration with computerized maintenance management systems (CMMS), quality assurance databases, or regulatory compliance documentation. Digital record-keeping eliminates transcription errors from manual recording and provides audit trails for accreditation surveys. Stored data includes test date, device serial number, test parameters, results, and pass/fail status—complete documentation for regulatory compliance.
Why Choose MS200 NIBP Simulator?
Professional NIBP Testing Standard
The MS200 represents the gold standard for non-invasive blood pressure monitor testing, combining reference-grade accuracy with comprehensive functionality in a portable, user-friendly instrument. As healthcare facilities face increasing pressure to document medical device performance while controlling costs, the MS200 provides a cost-effective solution that meets or exceeds regulatory requirements for NIBP monitor verification.
Accuracy is the foundation of effective calibration, and the MS200’s ±1 mmHg precision provides confidence that test results reflect true NIBP monitor performance rather than simulator limitations. This accuracy level, traceable to NIST standards, satisfies the most stringent regulatory requirements including Joint Commission, FDA QSR, ISO 13485, and international medical device regulations. The precision pressure measurement system maintains calibration stability for extended periods, reducing the frequency and cost of simulator recalibration.
What distinguishes the MS200 from basic pressure calibrators is its ability to generate realistic oscillometric waveforms rather than just static pressures. Modern NIBP monitors use sophisticated algorithms to analyze oscillometric pulse envelopes and extract blood pressure values. Testing these monitors with static pressure alone verifies only the pressure transducer—not the complete measurement system. The MS200’s dynamic waveform simulation exercises the entire oscillometric analysis chain, revealing algorithm failures that static testing cannot detect.
The dual patient mode capability (adult and neonate) addresses a critical testing gap. Pediatric and neonatal NIBP monitors operate with different algorithms optimized for smaller pulse amplitudes and different compliance characteristics. Testing these specialized monitors with adult simulation parameters produces misleading results. The MS200’s dedicated neonate mode ensures accurate verification of pediatric monitors used in NICUs, PICUs, and pediatric wards—critical for patient safety in vulnerable populations.
Arrhythmia and artifact simulation capabilities test NIBP monitors under the challenging conditions they face in actual clinical use. A monitor that performs perfectly with normal sinus rhythm may fail catastrophically when measuring blood pressure in a patient with atrial fibrillation or during patient movement. The MS200’s comprehensive artifact simulation identifies monitors with inadequate artifact rejection before they cause clinical problems, preventing false alarms, measurement failures, or worse—erroneous readings that mislead clinical decision-making.
The integrated pump and battery operation deliver genuine portability, transforming NIBP testing from a bench-only procedure to a truly mobile operation. Field service technicians can test monitors in situ without disconnecting and transporting them to workshops. Biomedical engineering departments can perform bedside verification during scheduled preventive maintenance rounds. This mobility accelerates testing workflows, reduces equipment downtime, and minimizes disruption to clinical operations.
Data storage and export functionality addresses modern quality assurance and regulatory compliance requirements. Manual paper records of NIBP testing are error-prone, difficult to search, and inadequate for trend analysis identifying deteriorating equipment before failure. The MS200’s digital record-keeping integrates with CMMS systems, providing searchable histories, automated compliance reporting, and analytics identifying devices requiring replacement based on performance trends rather than arbitrary age-based retirement policies.

Frequently Asked Questions
How often should NIBP monitors be tested with a simulator?
Testing frequency depends on regulatory requirements, manufacturer recommendations, and institutional policy. Most hospitals follow semi-annual or annual preventive maintenance schedules for NIBP monitors. The Joint Commission requires testing at intervals defined by manufacturer recommendations or hospital policy. Many manufacturers recommend annual calibration verification. High-risk clinical areas like operating rooms and intensive care units may warrant more frequent testing (quarterly or semi-annually).
Beyond scheduled preventive maintenance, test NIBP monitors after repair, before returning rental equipment, following any incident involving questionable readings, and when clinicians report measurement concerns. The MS200’s rapid testing capability makes it practical to perform verification checks whenever there’s any doubt about NIBP monitor accuracy. Document all testing in equipment maintenance records for regulatory compliance and quality assurance purposes.
Does the MS200 replace the need for mercury sphygmomanometer standards?
Yes, the MS200 provides modern electronic pressure measurement that eliminates mercury exposure hazards while delivering superior accuracy. Mercury sphygmomanometers, once the gold standard for blood pressure calibration, are being phased out worldwide due to environmental and health concerns about mercury. The MS200’s electronic pressure sensor, calibrated to NIST-traceable standards, provides ±1 mmHg accuracy matching or exceeding mercury column precision without toxic materials.
Electronic simulators offer significant advantages over mercury standards: no mercury disposal requirements, insensitivity to gravity and leveling (mercury columns require precise vertical positioning), automated testing reduces user technique variability, digital displays eliminate parallax reading errors, and comprehensive data logging capabilities. The MS200 represents the future of NIBP calibration as healthcare facilities transition away from mercury-based instruments in compliance with environmental regulations and green hospital initiatives.
Can the MS200 test all brands and models of NIBP monitors?
The MS200 is compatible with all oscillometric NIBP monitors regardless of manufacturer or model. The device simulates the physical properties (compliance, pulse amplitude) of an actual limb, not electronic signals, so it works with any monitor that uses standard pneumatic cuffs and oscillometric measurement technology. This includes monitors from major manufacturers like GE, Philips, Mindray, Welch Allyn, and many others.
The MS200 accommodates both arm and wrist cuffs through its standard connector. Different NIBP monitor brands may have varying pass/fail criteria or specific test protocols—consult each manufacturer’s service manual for acceptance thresholds when evaluating test results. The MS200’s flexibility in generating custom blood pressure values allows compliance with any manufacturer’s testing specifications. For aneroid (manual) sphygmomanometers using Korotkoff sounds rather than oscillometric detection, the MS200 can verify gauge accuracy but cannot simulate Korotkoff sounds.
How is the MS200 itself calibrated and how often?
The MS200 should be calibrated annually by a metrology laboratory with NIST-traceable pressure standards. Calibration verifies that the internal pressure sensor maintains ±1 mmHg accuracy specification across the entire operating range. A calibration certificate documenting traceability should be maintained with equipment records. Some regulatory frameworks require more frequent calibration verification (semi-annual) for critical measurement equipment.
Between formal calibrations, perform functional verification by testing a known-good NIBP monitor monthly and comparing results against baseline values. Significant deviations from expected values may indicate simulator drift requiring recalibration. Protect the MS200 from shock, drops, or extreme temperatures that could affect calibration. If the device is dropped or subjected to environmental extremes, perform verification testing or return to calibration service even if the annual interval hasn’t elapsed. Maintain calibration records as part of your quality assurance documentation for regulatory inspections.
What should I do if an NIBP monitor fails testing?
Document the failure with specific details: which test parameters failed, measured versus expected values, and test conditions. Immediately tag the monitor as “Out of Service” and remove it from clinical use—an inaccurate blood pressure monitor poses serious patient safety risks. Investigate whether recent clinical decisions were based on readings from the failed monitor; notify appropriate medical staff if any patient care concerns arise.
Common failure modes include: pneumatic leaks (replace tubing or valve), sensor calibration drift (requires manufacturer service), algorithm failures with arrhythmia (firmware update or replacement may be needed), and overpressure valve defects (immediate safety concern requiring repair before return to service). Some failures can be corrected in-house by biomedical technicians; others require manufacturer service. After repair, retest with the MS200 to verify correction before returning the monitor to clinical service. Track failure trends to identify models with chronic problems that may warrant replacement.
Is special training required to use the MS200?
The MS200’s intuitive interface minimizes training requirements, but users should understand basic blood pressure measurement principles and NIBP monitor operation. Biomedical equipment technicians (BMETs) typically require only 30-60 minutes of hands-on training to become proficient with the MS200. Training should cover: device setup and connections, selecting appropriate test modes (adult vs. neonate), interpreting test results and acceptance criteria, performing leak tests and safety valve verification, and data export procedures.
Written standard operating procedures (SOPs) should document your facility’s NIBP testing protocols, including which test scenarios to use, acceptance thresholds for pass/fail decisions, and documentation requirements. The MS200’s user manual provides detailed operation instructions and troubleshooting guidance. Periodic competency assessment ensures technicians maintain proficiency and follow established procedures correctly. While the device is simple to operate, understanding blood pressure physiology and NIBP monitor technology enables more effective troubleshooting when unexpected results occur.
Can the MS200 detect NIBP monitors that work but provide inaccurate readings?
Yes, detecting subtle inaccuracies is precisely the MS200’s primary purpose. Many NIBP monitor failures don’t cause obvious malfunctions—the device appears to work normally but provides readings offset from true values by 5-10 mmHg. These insidious errors are particularly dangerous because clinical staff have no indication that readings are wrong, potentially leading to inappropriate treatment decisions based on falsely high or low blood pressure values.
The MS200 quantifies measurement error by comparing the NIBP monitor’s displayed values against known reference pressures generated by the simulator. Testing at multiple pressure points (e.g., 90/60, 120/80, 160/100) reveals whether errors are constant across the range or vary with pressure level. Systematic offsets suggest calibration drift; pressure-dependent errors may indicate nonlinearity requiring more extensive repair. The arrhythmia simulation testing is equally important—it identifies monitors that function correctly under ideal conditions but fail to reject artifacts in challenging situations, potentially reporting dangerously incorrect values in patients with irregular heart rhythms.
What is included with the MS200 purchase?
The standard MS200 package includes: the main simulator unit, AC power adapter (100-240V), rechargeable battery (pre-installed), USB cable for data export, pressure connection tubing, carrying case for transport and storage, user manual with operating instructions, and initial calibration certificate documenting NIST-traceable accuracy. Some suppliers include additional accessories like test cuffs or adapters for connecting to various NIBP monitor brands.
Verify what’s included with your specific purchase—package contents may vary by supplier and region. The MS200 requires only standard NIBP cuffs (not included) to connect to monitors under test; use adult, pediatric, or neonatal cuffs as appropriate. Software for managing test data on computer is typically available as a free download from the manufacturer. Consider purchasing a spare battery for extended field work if your testing workflows involve many consecutive tests. Extended warranty or calibration service agreements may be available from authorized distributors.