Portable Bone Density Scanner NEUBD09
$2,790.00$2,950.00 (-5%)
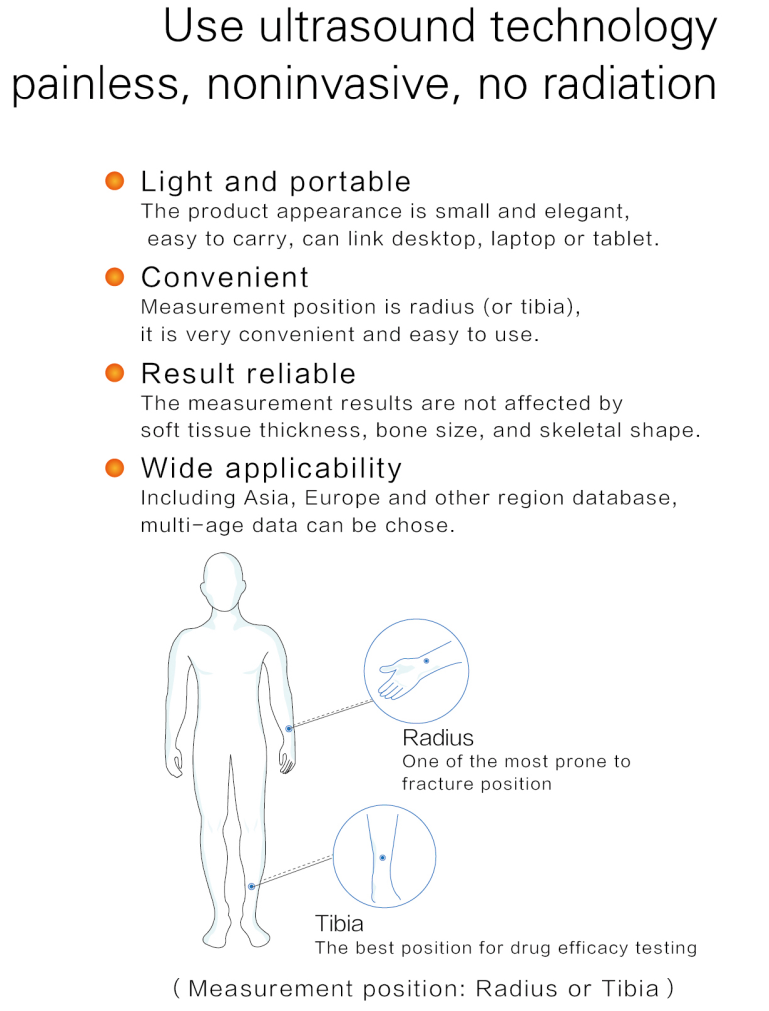
– Portable and lightweight design for easy transportation
– Non-invasive ultrasound technology for measuring bone density
– Suitable for pregnant women, children, and elderly populations
– Fast measurement time: single measurement in ≤ 25 seconds
– Provides reliable results unaffected by soft tissue thickness
– User-friendly operation linked to desktop computers, laptops, or tablets
– Multiple databases for diverse age groups across various regions
– Measures radius or tibia bone density for comprehensive analysis
– Compatible with Windows XP or higher operating systems
– Operates effectively in temperatures of 5-40℃ and up to 80% humidity
Portable Bone Density Scanner NEUBD09
Non-invasive ultrasound bone densitometry system for rapid osteoporosis screening. Portable design with critical angle side wave technology for accurate radius and tibia measurements.
FDA Approved
ISO 13485 Certified
2-Year Warranty
24/7 Support

Technical Specifications
| Specification | Details |
|---|---|
| Model Number | NEUBD09 |
| Measurement Sites | Radius or tibia (peripheral bone sites) |
| Measurement Technology | Critical Angle Side Wave Theory |
| Ultrasound Technology | Non-invasive quantitative ultrasound |
| Probe Frequency | 1 MHz ±15% |
| Measurement Parameters | SOS, T-score, Z-score, Skeletal Age, BQI, RRF, EOA |
| Single Measurement Time | ≤ 25 seconds |
| Repetition Time | ≤ 75 seconds between measurements |
| Operating System | Windows XP or higher |
| Reference Database | Multi-age data from Asia, Europe, and other regions |
| Soft Tissue Independence | Results not affected by soft tissue thickness |
| Operating Temperature | 5°C – 40°C |
| Operating Humidity | ≤ 80% relative humidity |
| Power Requirements | AC 220V ±10%, 50Hz ±1Hz |
| Design | Portable and lightweight for easy transportation |
| Warranty | 2-year manufacturer warranty |
Measurement Parameters Explained
Comprehensive bone health assessment parameters
| Parameter | Description |
|---|---|
| SOS (Speed of Sound) | Primary measurement parameter indicating bone density through ultrasound velocity |
| T-score | Comparison to peak bone mass of healthy young adults (WHO diagnostic criteria) |
| Z-score | Comparison to age-matched reference population for pediatric and young adult assessment |
| Skeletal Age | Estimated bone age based on bone density measurements |
| BQI (Bone Quality Index) | Overall bone quality assessment combining multiple parameters |
| RRF (Relative Risk Factor) | Fracture risk assessment relative to reference population |
| EOA (Expected Osteoporotic Age) | Predicted age at which osteoporosis may develop based on current bone density |
Clinical Applications
Versatile bone density screening for diverse patient populations
Key Benefits & Features
Radiation-Free Technology
Non-invasive quantitative ultrasound eliminates radiation exposure, making it safe for repeated measurements, pediatric patients, pregnant women, and individuals requiring frequent monitoring. No special room shielding or radiation safety protocols required.
- Zero radiation exposure to patients and staff
- Safe for pediatric and pregnant populations
- Suitable for frequent serial measurements
- No radiation safety infrastructure needed
Rapid Measurement Time
Complete bone density scan in 25 seconds or less with repeat measurements possible in 75 seconds. This efficiency enables high-throughput screening programs and minimizes patient waiting time in busy clinical settings.
- ≤25 seconds single measurement time
- ≤75 seconds between repeat measurements
- High patient throughput capability
- Minimal disruption to clinic workflow
Portable & Lightweight Design
Compact portable design enables bone density screening in multiple locations including physician offices, nursing homes, health fairs, and mobile clinics. Easy transportation between sites expands service reach and revenue opportunities.
- Portable design for multi-site use
- Easy transportation between locations
- Suitable for mobile screening programs
- No facility infrastructure modifications needed
Critical Angle Side Wave Technology
Advanced measurement technology based on critical angle side wave theory provides accurate bone density assessment at peripheral sites. This methodology correlates well with central DXA measurements for osteoporosis risk assessment.
- Advanced ultrasound measurement technology
- Accurate peripheral bone density assessment
- Correlation with central DXA measurements
- Validated for osteoporosis screening
Suitable for All Populations
Safe and appropriate for pregnant women, children, elderly patients, and all age groups requiring bone density assessment. Multi-age reference database from diverse geographic regions ensures accurate interpretation across populations.
- Safe for pregnant women and children
- Appropriate for elderly populations
- Multi-age reference databases included
- Diverse geographic population norms
Soft Tissue Independence
Measurement results are not affected by soft tissue thickness, bone size, or skeletal shape variations. This independence from anatomical factors improves measurement reliability across diverse patient body types and reduces potential sources of error.
- Unaffected by soft tissue thickness
- Independent of bone size variations
- Reliable across diverse body types
- Consistent results regardless of anatomy
Comprehensive Parameter Report
Seven measurement parameters (SOS, T-score, Z-score, Skeletal Age, BQI, RRF, EOA) provide thorough bone health assessment. Multiple parameters enable comprehensive evaluation of bone density, quality, and fracture risk.
- 7 bone density parameters measured
- T-score and Z-score for WHO criteria
- Fracture risk assessment (RRF)
- Bone quality index and skeletal age
Radius & Tibia Measurement Sites
Peripheral bone site measurement at radius or tibia provides accessible, comfortable patient positioning. These sites are weight-bearing bones that reflect overall skeletal health and osteoporosis risk.
- Radius and tibia measurement capability
- Easy patient positioning
- Weight-bearing bone assessment
- Reflects overall skeletal health
Windows-Based Software
Familiar Windows XP or higher operating system simplifies operator training and system integration. Standard computer interface enables easy data management, report generation, and electronic medical record connectivity.
- Windows XP or higher compatibility
- Familiar user interface
- Easy operator training
- Standard EMR connectivity options
Point-of-Care Testing
Immediate results at point of care enable same-visit patient counseling and treatment decisions. Eliminates the delay and inconvenience of referring patients to imaging centers for DXA scans.
- Immediate results during patient visit
- Same-day counseling and treatment planning
- No referral to imaging center needed
- Improved patient satisfaction and compliance
Cost-Effective Screening
Lower equipment and operating costs compared to DXA systems make bone density screening accessible for smaller practices and screening programs. No radiation safety infrastructure or specialized facility requirements reduce overhead expenses.
- Lower capital investment than DXA
- Reduced operating costs
- No radiation safety infrastructure needed
- Accessible for smaller practices
Multi-Region Reference Database
Comprehensive reference databases from Asia, Europe, and other regions ensure accurate interpretation for diverse patient populations. Multi-ethnic normative data improves diagnostic accuracy across different demographic groups.
- Asian reference database included
- European reference database included
- Multi-ethnic population norms
- Accurate interpretation across demographics
Frequently Asked Questions
How does quantitative ultrasound compare to DXA for bone density measurement?
Quantitative ultrasound (QUS) and DXA measure different aspects of bone health. DXA remains the gold standard for osteoporosis diagnosis, measuring bone mineral density at central sites (spine and hip). QUS measures bone quality parameters like speed of sound at peripheral sites (radius/tibia) and provides fracture risk assessment. QUS offers advantages including no radiation, lower cost, portability, and suitability for screening. Patients with abnormal QUS results can be referred for confirmatory DXA scanning.
Can I use this device to diagnose osteoporosis?
The NEUBD09 is intended for osteoporosis screening and fracture risk assessment rather than definitive diagnosis. While T-scores are provided for clinical interpretation, WHO diagnostic criteria for osteoporosis are based on DXA measurements of spine or hip. The device identifies patients at risk who should be referred for DXA confirmation and those who may benefit from preventive interventions. It’s excellent for screening large populations and monitoring treatment response.
Why measure at radius or tibia instead of spine or hip?
Peripheral sites (radius and tibia) are more accessible and easier to measure with ultrasound technology. These weight-bearing bones correlate with overall skeletal health and fracture risk. The measurement is quick, comfortable for patients, and doesn’t require special positioning. While central sites (spine/hip) are preferred for diagnostic purposes, peripheral measurements provide valuable screening information and predict fracture risk. Radius and tibia measurements are particularly useful when central site scanning is not available or practical.
Is any special training required to operate the device?
Basic operator training can be completed in 1-2 hours. The Windows-based interface is intuitive, and probe positioning is straightforward. We provide comprehensive training during installation covering patient positioning, probe placement, quality control, result interpretation, and system maintenance. Medical assistants, nurses, or technicians can easily learn to perform scans. While operation is simple, result interpretation should be performed by qualified healthcare providers familiar with bone density assessment.
How often should patients be screened?
Screening frequency depends on individual risk factors and initial results. Generally, postmenopausal women and elderly individuals may benefit from screening every 1-2 years. Patients on osteoporosis treatment should be monitored according to treatment protocols, often annually. Because ultrasound is radiation-free, more frequent monitoring is possible when clinically indicated. Your clinical judgment and established guidelines (USPSTF, NOF) should guide screening intervals for your patient population.
Can results be exported or integrated with EMR systems?
The Windows-based system allows report generation and data export. Results can be printed for patient records or saved as digital files for EMR integration. Standard connectivity options facilitate data transfer to electronic medical record systems. Contact us to discuss your specific EMR requirements and integration capabilities. We can provide technical specifications for IT departments to assess compatibility with your existing systems.
What maintenance does the device require?
The NEUBD09 requires minimal routine maintenance. Daily tasks include system calibration checks and probe cleaning. Periodic quality control measurements with phantoms ensure measurement accuracy. The ultrasound probe should be handled carefully and protected from damage. No consumables are required for routine operation. Annual preventive maintenance by a service technician is recommended to verify system performance and calibration. The Windows computer requires standard antivirus and software updates.
What warranty and support is provided?
The NEUBD09 includes a 2-year manufacturer warranty covering parts and labor. Neuvar provides 24/7 technical support at +1 424 270 9077 and sales@neuvar.com for troubleshooting and questions. We maintain parts inventory for rapid service response and can arrange extended warranty programs. Remote technical support via phone or screen sharing resolves most issues quickly. On-site service is available when needed. Software updates and patches are provided throughout the warranty period.
Add Bone Density Screening to Your Practice Today
Expand your services with radiation-free, portable bone density technology.
Contact Our Team

| Measurement position | Radius (NEUBD09-NEUBD10) |
| Measurement theory | Critical Angle Side Wave Theory |
| Ultrasound Parameters | SOS, T-score |
| Other parameters | T-score, Z-score, Skeletal age, BQI, RRF, EOA |
| Probe frequency | 1MHz±15% |
| Measure time | Single ≤ 25s, Repettion ≤ 75s |
| Operation system | windows XP or above |
| Operating temperature | 5-40℃ |
| Operating humidity | Relative humidity ≤ 80% |
| Power requirement | AC220V±10%,50Hz±1Hz |
| Suitable population | Pregnant women, Baby, Children, The elderly |