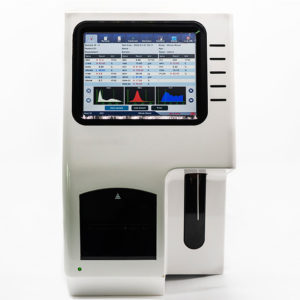
Chemiluminescence Immunoassay ECLIA Analyzer NEUCM12
$33,750.00$47,000.00 (-28%)
– High-performance Chemiluminescence Immunoassay Analyzer for accurate diagnostics
– User-friendly touch screen with Smart QC and Calibration Management
– Supports multiple assays with a capacity for 30 samples and 100 incubation positions
– Fast assay duration of 18 minutes per test, processing up to 86 tests per hour
– Compact design: Dimensions 650(H) × 620(W) × 650(D) mm, Weight: 92 kg
– Monitors dynamic IgM & IgG titer changes, essential for COVID-19 stage determination
– No interference from common viruses including Influenza, HCV, and HBV
– Includes various consumables: buffers, reagents, and assay cups for uninterrupted operation
– Ideal for clinical laboratories and rapid testing environments
– Robust and reliable technology tailored for high-throughput testing needs.
Chemiluminescence Immunoassay ECLIA Analyzer NEUCM12
High-Performance Automated Immunoassay System for Precise Clinical Diagnostics
Free Worldwide Shipping
CE & FDA Approved
Medical Grade Quality








Advanced Chemiluminescence Technology for Laboratory Excellence
The NEUCM12 Chemiluminescence Immunoassay ECLIA Analyzer represents the pinnacle of automated immunoassay technology, delivering exceptional sensitivity and specificity for clinical diagnostics. This high-performance system combines rapid throughput with intelligent quality control features, making it an indispensable tool for modern clinical laboratories.
Engineered with cutting-edge chemiluminescence detection technology, the NEUCM12 provides reliable quantitative analysis across a wide range of biomarkers including hormones, tumor markers, infectious disease markers, and cardiac markers. The system’s intuitive touch screen interface and smart calibration management ensure consistent, accurate results while minimizing operator intervention and streamlining workflow efficiency.
Clinical Applications
Technical Specifications
| Specification | Details |
|---|---|
| Detection Method | Chemiluminescence Immunoassay (ECLIA) |
| Sample Capacity | 30 positions (refrigerated) |
| Incubation Positions | 100 reaction cuvettes |
| Reagent Channels | 10 onboard refrigerated positions |
| Throughput | Up to 86 tests per hour |
| Assay Duration | 18 minutes per test (first result) |
| Sample Volume | 10-100 μL (programmable) |
| Reagent Volume | 100-300 μL per test |
| Calibration Stability | 28 days (varies by parameter) |
| Quality Control | Automatic QC with Levey-Jennings charts |
| Display | 10.4-inch color touch screen interface |
| Data Storage | Up to 500,000 test results |
| Connectivity | LIS/HIS bidirectional interface, USB, Ethernet |
| Dimensions (H×W×D) | 650 × 620 × 650 mm |
| Weight | 92 kg |
| Power Requirements | AC 100-240V, 50/60 Hz, 500 VA |
| Operating Environment | 15-30°C, relative humidity 30-80% (non-condensing) |
| Barcode Reader | Integrated automatic sample and reagent barcode scanning |
Comprehensive Test Menu
| Category | Available Tests |
|---|---|
| Infectious Disease Markers | COVID-19 IgM/IgG, HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, HCV Ab, HIV Ag/Ab, TP, Rubella IgM/IgG |
| Tumor Markers | AFP, CEA, CA125, CA19-9, CA15-3, PSA, free PSA, CYFRA 21-1, NSE, SCC |
| Cardiac Markers | cTnI, CK-MB, Myoglobin, BNP, NT-proBNP, D-Dimer |
| Thyroid Function | T3, T4, FT3, FT4, TSH, Anti-TPO, Anti-TG |
| Fertility Hormones | FSH, LH, PRL, E2, PROG, β-HCG, Testosterone |
| Diabetes Markers | Insulin, C-peptide, HbA1c |
| Bone Metabolism | PTH, 25-OH Vitamin D, Calcitonin |
| Anemia Markers | Ferritin, Transferrin, Vitamin B12, Folic Acid |
Key Features and Benefits
Advanced Chemiluminescence Technology
Utilizes state-of-the-art chemiluminescent detection with enhanced sensitivity and specificity, achieving detection limits in the pg/mL range for most analytes. The system’s optimized signal amplification ensures reliable quantification even at low biomarker concentrations.
High Throughput Performance
Processes up to 86 tests per hour with first results available in just 18 minutes, dramatically reducing turnaround time and increasing laboratory efficiency. The continuous loading capability allows uninterrupted operation during high-volume testing periods.
Intuitive Touch Screen Interface
Features a 10.4-inch high-resolution color touch screen with user-friendly software interface. Real-time monitoring of all system functions, graphical display of QC results, and comprehensive patient data management enhance operator convenience and workflow efficiency.
Smart Quality Control Management
Automated quality control with intelligent Levey-Jennings chart generation, Westgard rule violation detection, and automatic reagent and calibration expiration alerts. The system ensures compliance with clinical laboratory quality standards while minimizing manual QC tasks.
Automatic Barcode Recognition
Integrated barcode readers for both samples and reagents eliminate manual entry errors and ensure proper sample identification. The system automatically tracks reagent lot numbers, expiration dates, and remaining test volumes.
Onboard Refrigeration System
Refrigerated sample and reagent storage maintains optimal temperature conditions (2-8°C) for extended reagent stability and sample integrity. The system’s intelligent temperature monitoring provides continuous protection against thermal excursions.
Comprehensive Connectivity
Bidirectional LIS/HIS interface enables seamless integration with hospital information systems for automated test ordering, result transmission, and quality control documentation. USB and Ethernet connectivity provide flexible data export and remote monitoring capabilities.
Efficient Reagent Management
Ten onboard reagent positions with automatic inventory tracking reduce manual reagent handling. The system alerts operators to low reagent volumes and provides accurate remaining test counts, preventing test interruptions due to reagent depletion.
Extensive Data Storage
Internal memory stores up to 500,000 test results with complete audit trails, calibration curves, and quality control data. Advanced search and filtering capabilities facilitate rapid data retrieval for patient history review and regulatory audits.
No Cross-Reactivity
Validated to show no interference from common viruses including Influenza A/B, HCV, HBV, respiratory syncytial virus, and various coronavirus strains. This ensures accurate COVID-19 IgM/IgG testing and reliable results for infectious disease panels.
Environmental Efficiency
Low reagent consumption and minimal waste generation reduce environmental impact and operating costs. The system’s energy-efficient design and standby mode minimize power consumption during idle periods.
Easy Maintenance
Automated cleaning cycles, self-diagnostic routines, and modular design simplify preventive maintenance. The system provides clear maintenance reminders and step-by-step guidance for routine service procedures, minimizing downtime.
Why Choose the NEUCM12 ECLIA Analyzer?
The NEUCM12 Chemiluminescence Immunoassay Analyzer delivers unmatched versatility and reliability for clinical laboratories seeking to enhance diagnostic capabilities while optimizing operational efficiency. This advanced system combines exceptional analytical performance with intelligent automation features that streamline workflow and reduce operator burden.
With its comprehensive test menu spanning infectious diseases, tumor markers, cardiac markers, hormones, and specialty assays, the NEUCM12 eliminates the need for multiple analyzer platforms, reducing equipment costs and consolidating training requirements. The system’s rapid 18-minute turnaround time and high throughput capacity ensure timely results for critical clinical decisions.
Smart quality control management with automatic calibration tracking, Westgard rule monitoring, and electronic documentation ensures compliance with regulatory standards while minimizing manual QC tasks. The bidirectional LIS/HIS connectivity facilitates seamless integration into existing laboratory information systems, enabling automated test ordering and result reporting.
Backed by comprehensive technical support, application training, and validated reagent kits, the NEUCM12 provides laboratories with a complete immunoassay solution. Whether upgrading existing capabilities or establishing new testing services, the NEUCM12 delivers the performance, reliability, and efficiency modern clinical laboratories demand.
Frequently Asked Questions
What is chemiluminescence immunoassay (ECLIA) technology?
Chemiluminescence immunoassay is an advanced diagnostic technique that uses light emission from chemical reactions to detect and quantify specific biomarkers. In ECLIA, antibodies labeled with chemiluminescent compounds bind to target analytes, and the resulting light emission is measured to determine concentration. This technology offers superior sensitivity compared to traditional enzyme immunoassays, enabling detection of biomarkers at very low concentrations (pg/mL to ng/mL range). The NEUCM12 utilizes optimized chemiluminescent reagents and advanced photon detection systems to achieve exceptional analytical performance across a wide dynamic range.
How many different tests can the NEUCM12 perform?
The NEUCM12 supports a comprehensive menu of over 80 different immunoassay tests across multiple clinical categories including infectious disease markers (hepatitis, HIV, COVID-19), tumor markers (AFP, CEA, PSA, CA125), cardiac markers (troponin, BNP, CK-MB), thyroid function tests, fertility hormones, diabetes markers, bone metabolism markers, and anemia markers. The system can run different test types simultaneously, allowing laboratories to efficiently process diverse sample workloads. New test parameters can be added as validated reagent kits become available, providing flexibility to expand testing capabilities as clinical needs evolve.
What is the calibration stability and how often does calibration need to be performed?
Calibration stability varies by test parameter but typically ranges from 14 to 28 days depending on the specific analyte. The NEUCM12’s smart calibration management system automatically tracks calibration expiration dates and alerts operators when recalibration is required. The system stores calibration curves electronically and automatically applies the appropriate curve for each test. Lot-to-lot reagent recalibration is required when new reagent lots are placed onboard. The analyzer’s refrigerated reagent storage and optimized chemistry formulations contribute to extended calibration stability, reducing the frequency of calibration procedures and minimizing reagent consumption.
Can the analyzer integrate with our existing Laboratory Information System (LIS)?
Yes, the NEUCM12 features comprehensive bidirectional LIS/HIS connectivity supporting standard communication protocols including HL7 and ASTM. The system can receive electronic test orders from your LIS, automatically process samples, and transmit results back to the LIS without manual data entry. This integration eliminates transcription errors, accelerates result reporting, and provides complete electronic documentation for regulatory compliance. The analyzer also supports remote monitoring and data export via Ethernet and USB connectivity. Our technical team can assist with interface configuration and validation to ensure seamless integration with your specific LIS platform.
What sample types can be analyzed on the NEUCM12?
The NEUCM12 is designed to analyze serum, plasma, whole blood, urine, and other biological fluids depending on the specific test parameter. For most immunoassay tests, serum or plasma are the preferred sample types. The analyzer’s refrigerated sample storage maintains sample integrity during the analysis process. Sample volumes are minimal, typically requiring only 10-100 μL per test, which is particularly advantageous for pediatric samples or when sample availability is limited. The system’s automated sample probe includes collision detection and liquid level sensing to ensure accurate sample aspiration and prevent cross-contamination between samples.
How does the quality control system work?
The NEUCM12 implements comprehensive automated quality control following Clinical Laboratory Improvement Amendments (CLIA) guidelines and Westgard QC rules. The system automatically runs quality control samples at user-defined intervals and generates Levey-Jennings charts to visualize QC performance over time. Multi-rule violation detection (1-2s, 1-3s, 2-2s, R-4s, 4-1s, 10x) identifies systematic errors, random errors, and trend patterns. When QC failures occur, the system automatically flags affected patient results and alerts operators to take corrective action. All QC data is stored electronically with complete audit trails for regulatory compliance and laboratory accreditation requirements.
What maintenance is required for the NEUCM12 analyzer?
The NEUCM12 is designed for minimal maintenance with automated daily, weekly, and monthly maintenance routines. Daily maintenance includes automated washing cycles and system diagnostics that run automatically. Weekly tasks involve waste container emptying and replenishment of washing buffer, which takes approximately 10 minutes. Monthly maintenance includes cleaning of sample and reagent probes and verification of system performance using quality control materials. The analyzer provides on-screen maintenance reminders with step-by-step instructions. Major preventive maintenance (cleaning of optical components, replacement of tubing and seals) is recommended every 6-12 months and can be performed by trained laboratory personnel or our service team. The modular design allows quick component replacement to minimize downtime.
What training and support is provided with the NEUCM12?
Comprehensive training and support is included with every NEUCM12 installation. Our factory-certified application specialists provide on-site installation, operator training (typically 2-3 days), and validation support to ensure proper system operation. Training covers instrument operation, quality control procedures, troubleshooting, routine maintenance, and LIS connectivity. We provide detailed user manuals, quick reference guides, and video tutorials for ongoing reference. Technical support is available via phone, email, and remote diagnostic connectivity to assist with any operational questions or technical issues. Annual preventive maintenance contracts are available to ensure optimal long-term performance. We also offer continuing education programs to keep your laboratory staff updated on new test parameters and advanced system features.
Contact Our Team